Using a digital validation lifecycle management system (VLMS) can save a company millions of dollars in project costs, increase productivity, and provide a host of other advantages that paper-based validation doesn’t offer. Chances are, if you’re reading this blog post, you’re well aware of the headaches of trying to manage your validation activities on paper using manual processes. What you may not know is why having a purpose-built VLMS is so important and how it differs from a document management system (DMS) and other solutions commonly found in the tech stacks of life sciences organizations.
I’ve asked Emmanuel Cansino, ValGenesis’ Senior Director of Industry Solutions, to shed some light on the subject. Here’s what he had to say.
Q: Emmanuel, for those unfamiliar with a DMS, can you define what it is and isn’t?
A: DMS is a system to receive, track, and manage electronic documents. While the DMS may contain historical validation execution records and evidence, they are simply stored there; the DMS does not manage the validation process. Customers often assume they can digitize their validation activities using a DMS, but this isn’t the case. A DMS can manage some aspects of validation, primarily document authoring, but it’s very limited.
Q: Besides storing and authoring documents, what other validation-related activities do people try to perform using a DMS?
A: Some people use it to approve their traceability matrices, although the generation, i.e., the actual mapping and tracing of user requirements with test cases, is done manually in Excel. Some might load their test executions after they scan them as PDF, but once again, that’s all manually driven, and it’s just the reviews and approvals.
Q: Can you talk more about building trace matrices in a DMS vs. a VLMS?
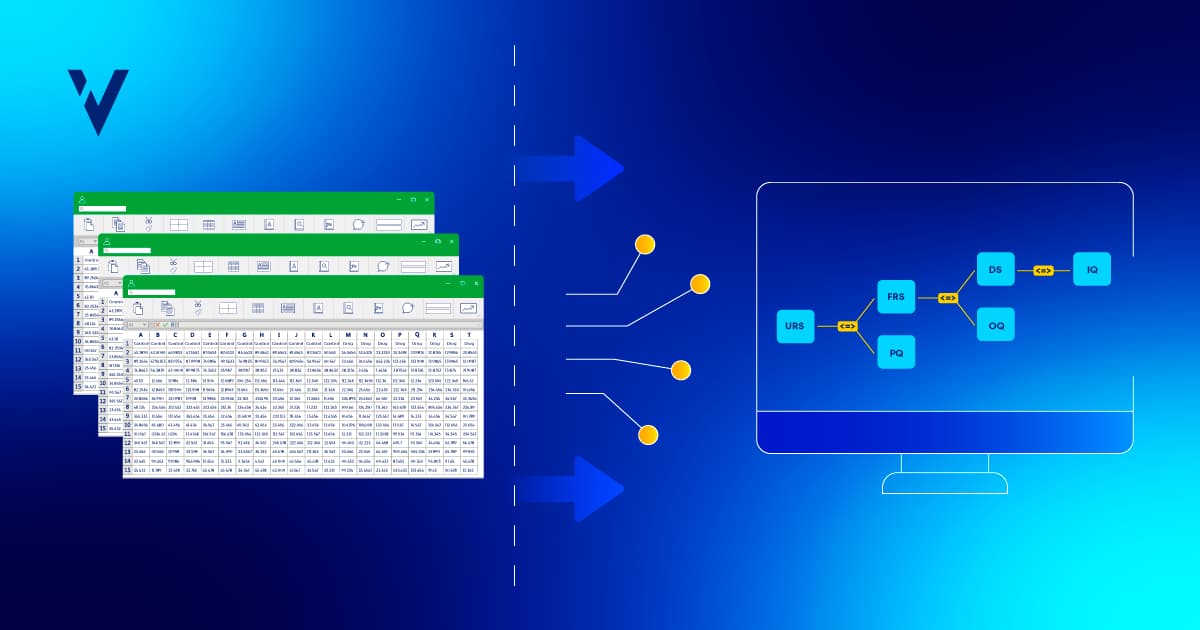
A: Typically, a validation engineer will write their requirements and tests, execute their tests, and then build the trace matrix in Excel after the tests have already been approved. Next, they’ll load the Excel document into the DMS and route it for review and approval. The process isn’t dynamic; there is no building of relationships between related items or dynamic links. The trace matrix isn’t automatically updated if there is a test failure. The DMS is just a repository for the trace matrix document; you can’t build a trace matrix in a DMS.
In our system, traceability matrices can be dynamically created and updated in real time to reflect any changes made to requirements or test functions. Users can map multiple documents against each other, automatically linking individual specifications to test step levels. Users can also view the traceability between documents for completeness before routing them for review and approval in a controlled workflow. This significantly reduces the overall time spent authoring documents.
Q: Can a VLMS replace a DMS?
A: No, each system serves a different purpose. A DMS is an excellent tool for managing documents, but it doesn’t offer the content generation capabilities our VLMS offers. For example, with our decision tree functionality, users answer questions, and based on their answers, the system automatically determines the content that needs to be populated in a particular document. Users can also load tags into templates, and those tags will extract information from the database as it relates to the documents being offered. So, with our VLMS, you’re not only saving time but also enforcing consistency in your content authoring processes.
Q: Can a document be authored in a DMS, then executed in ValGenesis VLMS?
A: Yes, but given the strength of our document authoring capabilities, I don’t see why anyone would want to. I’ve worked with many customers who initially asked that question, but once they see what they can do in ValGenesis, and how different it is, they opt to use ValGenesis to author all their validation documentation. They also realize that authoring within the ValGenesis platform becomes part of managing the data, the audit trail, the collaboration, etc., so it makes sense to do everything in one system.
Some customers want to integrate so they can author and execute in ValGenesis but store their validation documents in their DMS. In that case, the approved document is “pushed” from ValGenesis into the DMS, where it resides as the primary or binding copy, i.e., the record they would show to auditors. But again, most customers opt to use ValGenesis as their system of record because historical validation documents can be challenging to find quickly in a DMS.
Integrations are possible through our robust API stack. Generally, the integrations work together. We don’t replace anything they do, and they cannot replace anything we do.
Q: What other functions can you perform in a VLMS that can’t be performed in a DMS?
A: Electronic execution, change management, risk assessments, systems assessments, and schedule management – our VLMS offers cradle-to-grave management of anything and everything validation-related. From your initial commissioning and qualification activities through the qualification process to change management, and finally to retirement.
If you think of validation as a pie, document authoring, which is really the only overlapping functionality between a DMS and a VLMS, is 25% of the pie. A DMS can’t address the other 75% of validation activities ValGenesis supports.
If you'd like to hear more from Emmanuel, watch his informative webinar "Understanding Digital Validation: How Does It Work?"