Validation Doesn’t Need to Bleed Time or Money
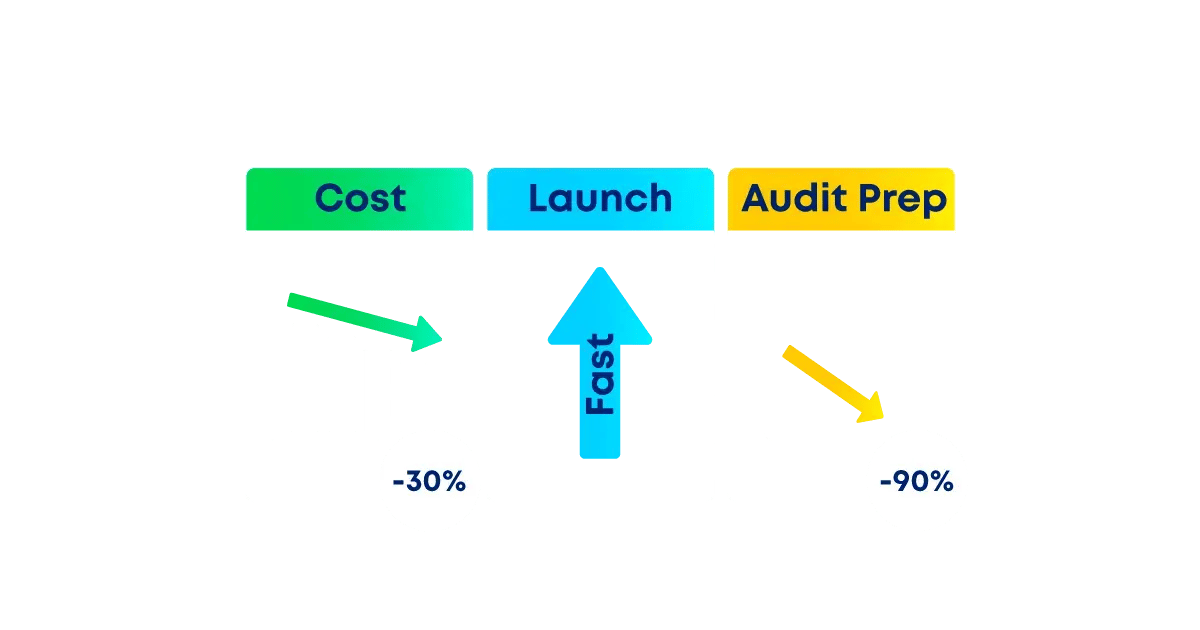
AI agents reduce manual validation efforts, cut costs by up to 30%, get new products out the door faster, and enable seamless regulatory inspections.
Challenges
Manual documentation consumes critical resources. Manual protocol creation devours skilled hours.
Cost overruns with every scale‑up. More sites = exponentially higher validation headcount.
Rework destroys schedules. Documentation gaps or data issues trigger costly do-overs and delays.
Slow validation stalls revenue. Launches wait while paperwork crawls.
The Lean, AI‑Powered Audit‑Ready Engine Your CQV Lacks
Paper‑heavy or siloed digital workflows keep validation teams locked in a grind: templates are rewritten, evidence is retyped, and every protocol revision triggers another paper trail. When audits arise, teams are forced into reactive efforts to resolve incomplete or missing records. The price? Lost weeks, escalating costs, and credibility risks.
What’s needed is a single, AI-enabled system that automates validation end-to-end―generating protocols from existing documents and enterprise data, capturing results through image-based verification, flagging exceptions for review, and analyzing content to detect gaps or GDP issues based on approved SOPs.
With AI agents, you eliminate rework, reduce manual oversight, and optimize resource use. Every record, including final approvals and signatures, is just one click away and always audit-ready. It’s lean, intelligent validation that keeps pace with your products.
ValGenesis—Performance Metrics You Can Bank On
ValGenesis Validation Lifecycle Suite delivers measurable impact:
- Cut Validation Costs by up to 30%—Millions in Annual Savings
Our AI streamlines document generation and execution, eliminating manual touchpoints and labor‑heavy rework.
- Accelerate Product Launches—Unlock Revenue Faster
Shorter validation cycles mean earlier approvals and quicker market entry.
- Boost the Bottom Line—Ensure Cost Efficiency
Control validation spend as you scale sites and products, keeping budgets tight and growth smooth.
Every action is digitally captured, timestamped, and evidence auto‑attached, reducing audit prep time by up to 90%. You walk into inspections calm—and walk out faster.
Empowering Life Sciences










Applications

iVal
Supercharge validation with ValGenesis iVal™. AI-powered authoring, automated execution, live anomaly flags, and bulletproof traceability slash cycles by up to 80% and cut observations by 90%. From CQV to CSA, you’ll be audit-ready and market-ready faster than ever. iVal takes digital validation to the next level.
Learn More
iClean
Halve cleaning validation timelines and wipe out manual math with ValGenesis iClean™. Automated MACO calculations, ADE-aligned limits, 2D/3D equipment maps, and rule-driven workflows deliver digital, inspection-ready files across all sites. Standardize globally, flex locally, and gain real-time oversight - discover iClean today.
Learn More
iOps
Leave paper logbooks behind with ValGenesis iOps™. Mobile, QR-enabled forms capture every use, cleaning, and calibration event in real time; automated reviews, deviations, and alerts seal compliance gaps. Achieve 100% traceability, trim documentation labor by 70%, and connect ops to quality with iOps today.
Learn MoreReady to Stop Bleeding Hours and Dollars on Validation—and Breeze Through Audits?
Book a 15‑minute demo and watch ValGenesis cut your CQV timelines.
Book a Demo