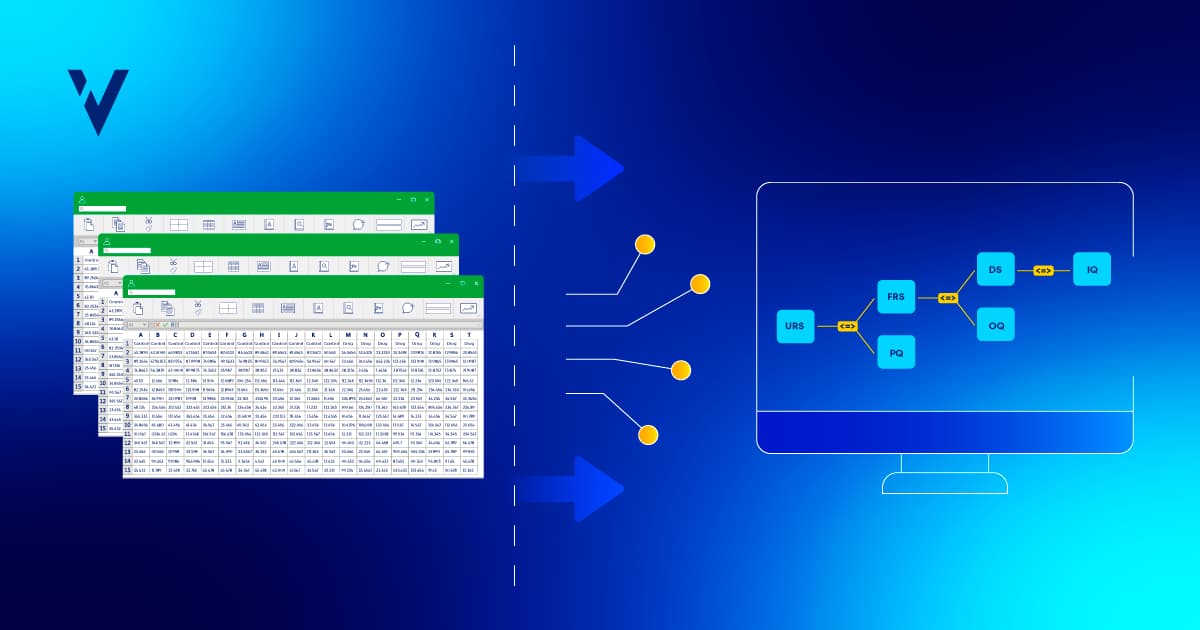
The Benefits of Automating Your Requirements Traceability Matrix
Automate your requirements traceability matrix to improve efficiency, reduce compliance risks, and streamline validation for better audit readiness.
Welcome to the ValGenesis blog, your resource for timely insights from industry-leading experts on the trends, best practices, and new technologies impacting the life sciences.
Jun 5, 2025 10:00:00 AM | Discover how digital cleaning validation strategies can modernize established pharma facilities, ensuring compliance, efficiency, and patient safety.
Discover how digital cleaning validation strategies can modernize established pharma facilities, ensuring compliance, efficiency, and patient safety.
Discover what cleaning validation is in pharma, why it's required, and how digital tools help you meet FDA cleaning validation guidelines efficiently.
Learn how spreadsheet chaos undermines Quality by Design in pharma—and how digital QbD platforms support CMC manufacturing and development.

AI-enabled digital validation can transform your processes, reducing errors, accelerating timelines, and ensuring compliance with evolving regulations.

Discover the hidden costs of manual CQV. Learn how AI-enabled digital CQV can enhance efficiency, compliance, and innovation in pharma manufacturing.
Discover how MilliporeSigma achieved rapid digital validation implementation with ValGenesis VLMS, transforming efficiency and compliance in just 97 days.

Automate your requirements traceability matrix to improve efficiency, reduce compliance risks, and streamline validation for better audit readiness.
Learn how Catalent scaled CSV compliance with ValGenesis VLMS to enhance global IT operations.

Learn how to build a compelling business case for transitioning to digital validation with four key conversations that drive stakeholder buy-in.

If you’re following these 3 core principles, odds are you’re already aligned with the FDA's computer software assurance (CSA) methodology.

Root-Cause Analysis
Finding the Root Cause in mAB Production: a ValGenesis Story
Learn how we helped a customer find the root cause of a problem in monoclonal antibody (mAB) production and become stronger.

Written by
Sofia Santos
Process Digitalization
How to Accelerate Process Scale-Up With Effective Tech Transfer
There's a need to accelerate pharma product development and process scale-up. Having an effective tech transfer strategy can help you.

Written by
Sofia Santos

Quality Risk Management
Risk and Data as Knowledge Enablers: a Lifecycle Approach - ValGenesis
Read the industry insight on the topic of Quality Risk Management

Presented by
Sandra Silva
CSA flips the paradigm of traditional CSV to "right-size" validation processes. Learn more about the benefits and principles of the CSA approach, which encourages critical thinking and automation.

Automate your requirements traceability matrix to improve efficiency, reduce compliance risks, and streamline validation for better audit readiness.
Previously
Catalent's Journey of Continuous Improvement for CSV Excellence
Are You Aligned with FDA's Computer Software Assurance Methodology?
Learn how digitization offers a consistent way to carry out cleaning validation lifecycle activities, ensuring your methods and procedures are risk-based and rooted in scientific evidence.

Discover how digital cleaning validation strategies can modernize established pharma facilities, ensuring compliance, efficiency, and patient safety.
Previously
Is Your Cleaning Validation Stuck in the Slow Lane?
Cleaning Validation Program Compliance: Build a Framework for Success
Waves of new technology and thinking — IoT, AR, cloud, Agile — are transforming the very foundation of validation processes. Learn how to leverage them in a Pharma 4.0 world.

Learn how Catalent scaled CSV compliance with ValGenesis VLMS to enhance global IT operations.
Previously
Validating Pharma 4.0 for Smart Manufacturing
The X Factor for Successful Digital Transformation in BioPharma: People
Join our newsletter to receive updates on the latest news and industry-related content tailored to your preferences.