Tech Transfers, or Technology Transfers, usually imply that you’re moving the manufacture of a drug from one facility, one scale or one drug lifecycle phase to another. Which is why they are so important. When you switch facilities, you want to transfer the knowledge and reproduce the process smoothly.
The Importance of Tech Transfer
There are, typically, three situations where you will need a Tech Transfer:
- Your drug is progressing through the lifecycle stages,
- You want to add capacity to your facility,
- You would like to transfer production to another site.
This makes having a proper tech transfer important to assure that your drug gets to market as fast as possible.
Other Situations Where You Might Need it
It might happen that you don’t have your own manufacturing capacities ready, your drug pipeline might be full or you want to bring the manufacturing capacity back in-house.
In these situations, you will be working with a Contract Manufacturing Organization (CMO) or a Contract Development and Manufacturing Organization (CDMO). And to work with them, you will need a proper Tech Transfer.
Breaking Down the Tech Transfer
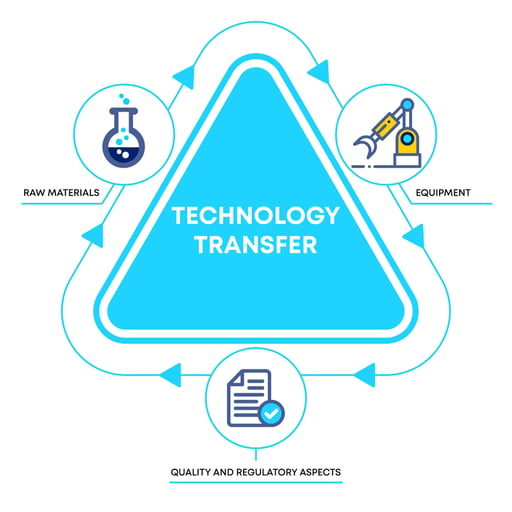
You can center Technology Transfer around three topics:
- Quality and Regulatory Aspects.
- Equipment.
- Raw Materials.
You will need to answer many questions such as:
- How different is the equipment and will that affect the process?
- Do you need to re-validate your process?
- Are your analytical methods still valid under a different regulatory environment?
- Will process adjustments be compatible with the equipment?
- If you need to replace them, will the new materials be compliant?
- Will you need to re-develop parts of the process?
And that’s just starting! You will need to cover many other points.
Making Sure Your Tech Transfer is Successful
When you perform a tech transfer, you want to make sure it’s successful! You want to avoid wasting time troubleshooting and you don’t want to risk the quality and efficiency of your process.
You want to start with the documentation. It needs to reflect product understanding as well as every relevant parameter and critical quality attributes. This is not an event as much as it is a practice. Good documentation supports consistent process execution.
You will need to include all the information related to Product, Process, Materials and Analytical Methods.
If you are working with a CMO/CDMO, you will also need a Tech Transfer Standard Operation Procedure (SOP). In it, you will define goals, scope, roles and responsibilities, tools, and communication procedures between the two companies.
But, most of all, a successful tech transfer implies understanding what both ends have for process and equipment. You need to know their differences and what you may need to re-develop.
Make sure that you have planned for success and for contingencies, and that you looked at the supply chain, the equipment, and the regulatory landscape.
Getting the Best Support
The best way to make sure your Tech Transfer yields the best results is to work with specialized consultants.
ValGenesis has a long and established expertise supporting world-class pharmaceutical companies with their Tech Transfers. We do this by using a Quality by Design (QbD) approach and Data-driven Process Design decisions.