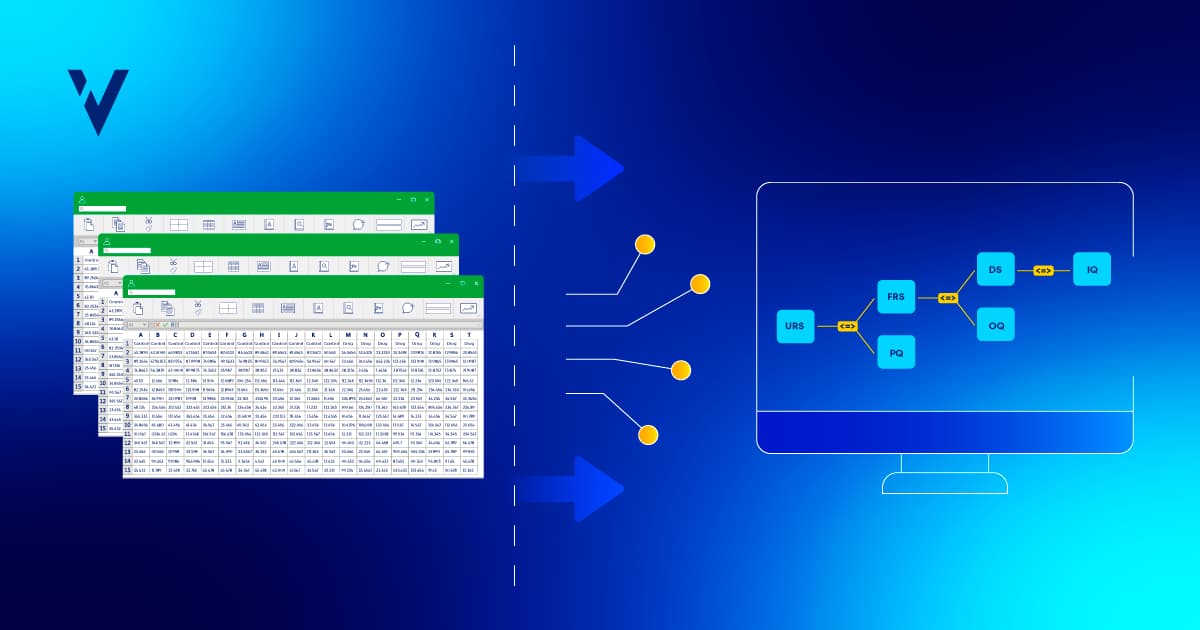
The Benefits of Automating Your Requirements Traceability Matrix
Automate your requirements traceability matrix to improve efficiency, reduce compliance risks, and streamline validation for better audit readiness.
Apr 12, 2023 11:10:11 AM | The second stage of the validation lifecycle is called Process Qualification. This stage is customary and is referred to as Cleaning Validation. Usually, three consecutive successful runs are performed to qualify the process using well characterized, well documented and consistent cleaning procedures.

Root-Cause Analysis
Finding the Root Cause in mAB Production: a ValGenesis Story
Learn how we helped a customer find the root cause of a problem in monoclonal antibody (mAB) production and become stronger.

Written by
Sofia Santos
Process Digitalization
How to Accelerate Process Scale-Up With Effective Tech Transfer
There's a need to accelerate pharma product development and process scale-up. Having an effective tech transfer strategy can help you.

Written by
Sofia Santos

Quality Risk Management
Risk and Data as Knowledge Enablers: a Lifecycle Approach - ValGenesis
Read the industry insight on the topic of Quality Risk Management

Presented by
Sandra Silva