In the highly competitive life sciences industry, being first to market is critical to success. Faster time to market means more revenue sooner, and accelerating the development and delivery of lifesaving therapies helps millions of patients.
Survival of the Fastest
The rise of automation, analytics, and disruptive technologies like artificial intelligence and the Internet of Things (IoT) have revolutionized the way we do business. And if the COVID-19 pandemic has taught us anything, it’s that nimbleness is no longer a quality reserved for startups — larger, more mature life sciences companies must become more responsive and agile in their practices to survive. The adoption of digital solutions is one way to do this.
The FDA recognizes the advantages of leveraging technology to accelerate time to market without compromising product quality or patient safety and is moving the industry towards more automated processes. Modern tools like a paperless validation lifecycle management system (VLMS) help regulated companies overcome the unique and difficult challenges that slow down their development and manufacturing processes.
What is Paperless Validation?
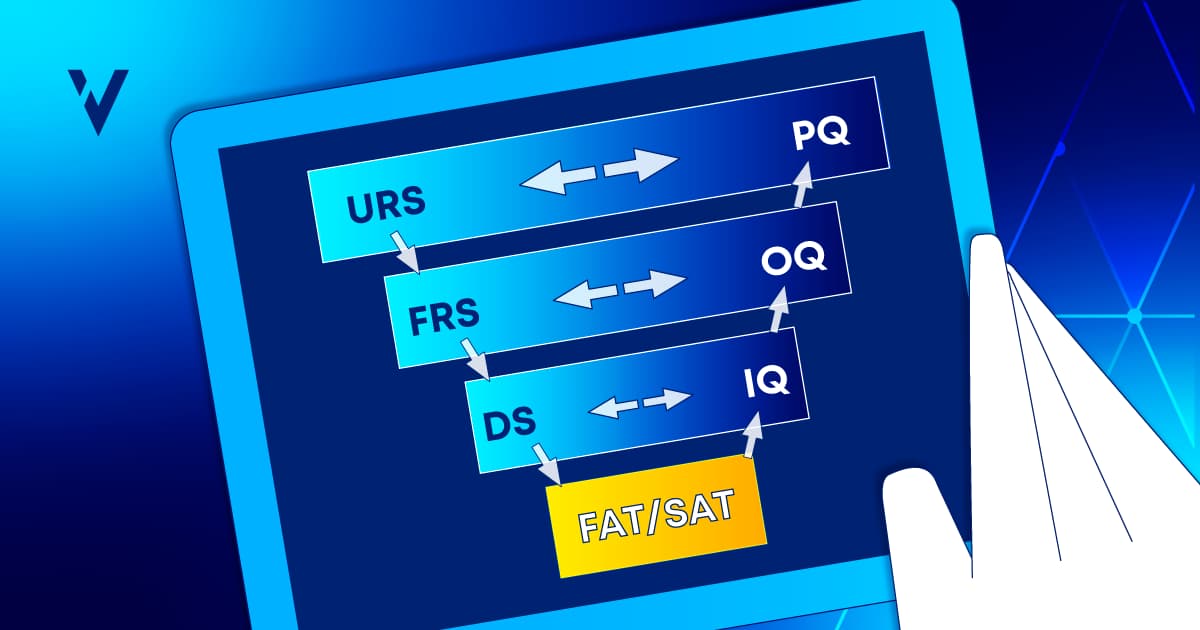
Paperless validation is the digital transformation of your existing paper-based validation processes through technology. A major step toward Pharma 4.0, paperless validation reduces the threat of human error and enables a deeper level of compliance through a solution that supports validated technical controls, automated workflows with predefined approval requirements, and enforced templates for consistency and quality.
Automating complex and time-consuming validation activities frees you from the burdens of paper-based validation such as poor collaboration, missing documents, expensive storage fees, information silos, inadequate audit trails…the list goes on and on. Poor paper processes lead to poor data quality which, according to Gartner, is responsible for an average of $15 million in losses every year. (1)
Validation consumes over 20% of any project budget due to the inherent inefficiencies of paper. Handling paper is costly from both a financial and time perspective. Paperless validation is a leap forward in efficiency; it can reduce the time it takes to complete validation projects by almost 50%, as our recent study revealed.
Real ROI Study
ValGenesis' Director of Industry Solutions Emmanuel Cansino surveyed eight ValGenesis customers, operating across pharma, medical device, and biotech, about their validation processes before and after implementing the ValGenesis VLMS. Participating companies ranged in size from $25 million to more than $I billion in annual revenue. The goal was to determine the savings and efficiency gains customers realized from digitizing their core validation activities with ValGenesis.
Customers were asked 37 process- and role-specific questions. Survey questions included:
- How many documents do you complete per year on average?
- How long does it take you to review and approve a document on average?
- How much time do you spend preparing for audits?
- What is the approximate salary for validation engineers?
Result Highlights
Based on the responses provided, we got a clear picture of the ROI of deploying an automated validation management solution like ValGenesis. A full breakdown of the survey parameters, results, and the formulas used to calculate the results are discussed in the 30-minute webinar, “How to (Really) Calculate the ROI of Digitized Validation.”
According to the results of the study:
- On average, clients spent $5,609.52 to author, execute, review and approve a document following traditional paper-based processes.
- On average, it took clients 33.28 business days to perform four core validation activities (content authoring, trace matrix design, execution, and deviation management) on one document using paper-based processes.
- By digitizing with ValGenesis, clients experienced a 50% overall efficiency gain on all validation processes, leading to faster time to market.
- By digitizing with ValGenesis, clients saw a 10% savings in project completion time, leading to faster time to market.
Making Your Business Case
By getting your products to market faster, you reduce the total cost of production and ultimately generate more revenue for your business. Using this webinar as a guide, you’ll learn which value metrics are important for calculating ROI and how to use data you already have to predict how much your organization could benefit from digitizing validation. Armed with this knowledge, you can communicate the value of digitizing to the business leaders in your company.
Watch the full webinar here.
- S. Moore, “How to Create a Business Case for Data Quality Improvement,” Garter.com, June 18, 2018.