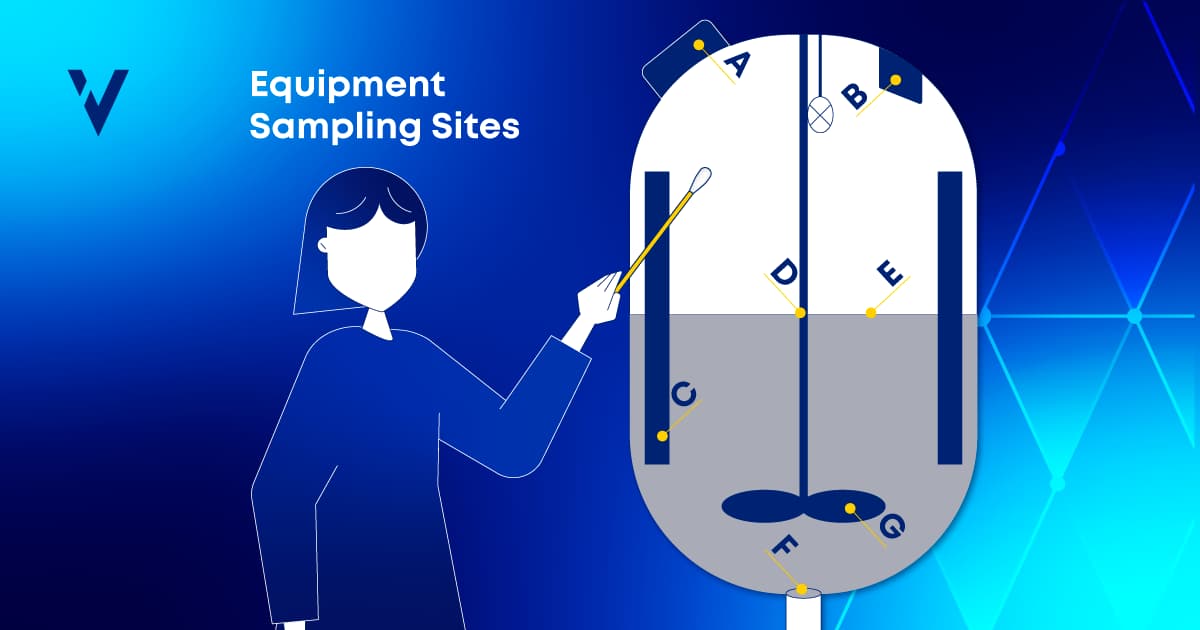
In the fast-paced world of pharmaceutical manufacturing, product quality and patient safety are paramount. Many facilities produce multiple drugs using shared equipment, so effective cleaning between product changeovers is essential. It helps you avoid cross-contamination and safeguards product identity, strength, quality, purity, and potency. This requirement puts enormous pressure on pharmaceutical manufacturers to uphold high cleaning standards to meet regulatory expectations.
A critical process in managing product changeovers is cleaning validation. Cleaning validation isn’t just a routine task; it’s a comprehensive, science-based approach that confirms your equipment is free from residues, contaminants, or trace amounts of active pharmaceutical ingredients (APIs) that could compromise product safety. The importance of robust cleaning validation systems increases when we consider the complex issues that can arise when transitioning from manufacturing product A to product B in shared environments.
In this blog post, we’ll examine the common issues encountered during product changeovers, the potential consequences of inadequate cleaning validation, and how implementing a digital cleaning validation solution can help you mitigate these risks and optimize operational availability.
Common Challenges in Product Changeovers
When pharmaceutical companies use shared manufacturing equipment for multiple products, the risks tied to ineffective cleaning validation can be significant. Some of the most relevant and persistent issues faced during product changeovers include:
- Residual APIs and cleaning agents: If cleaning validation and subsequent cleaning executions are not conducted correctly, residual APIs or cleaning agents may remain on the equipment. Minuscule amounts of these residuals can lead to product quality issues, regulatory noncompliance, and adverse patient effects, even within a single product campaign. During product changeovers, the risk of cross-contamination increases.
- Cross-contamination: Cross-contamination occurs when residues from one product inadvertently mix with another. This is particularly concerning for potent drugs or allergenic products, where even trace amounts can pose serious patient safety risks. Contaminated batches may require costly recalls and can damage your company’s reputation.
- Inconsistent cleaning procedures: One of the most common challenges in shared equipment environments is ensuring consistency in cleaning procedures. Manual processes and activities can vary between operators, leading to inconsistencies in the control of hold times, the application of cleaning agents, the duration and effectiveness of the cleaning, and the evaluation of results. This variability increases the risk of ineffective cleaning.
- Difficulty in monitoring and documentation: Manually managing cleaning validation often results in incomplete, error-filled documentation. It can create obstacles to accessing necessary information and tracking cleaning processes. Manual systems also make it difficult to evaluate the effectiveness of cleaning procedures over time, hindering continuous process control and improvement.
- Regulatory noncompliance: Regulatory bodies such as the FDA, EMA, and ICH have established strict cleaning validation guidelines to ensure the integrity of drug manufacturing processes. Failure to comply with these requirements can lead to warning letters, 483 observations, or, in the worst-case scenario, product recalls or legal actions.
Potential Consequences of Ineffective Cleaning Validation
Failing to address cleaning validation challenges during product changeovers can have serious repercussions. Ineffective cleaning validation may lead to:
- Patient safety risks: Residual contamination can cause unexpected pharmacological effects or severe allergic reactions in patients, jeopardizing drug safety.
- Product recalls: Contaminated products must be recalled, resulting in financial losses and damage to the manufacturer’s reputation.
- Production downtime: Inconsistent or faulty cleaning validation protocols can lead to extended equipment downtime while teams investigate and resolve contamination issues, reducing overall production capability.
- Regulatory scrutiny and penalties: Regulatory authorities enforce strict adherence to good manufacturing practices (GMPs) regarding cleaning validation. Noncompliance can lead to warning letters, production halts, or more severe enforcement actions. Historical issues may also trigger enhanced oversight and deeper scrutiny.
- Lost market trust: Failing to maintain product integrity can erode trust among consumers and healthcare providers, which could impact long-term profitability and brand credibility.
The Role of Digital Cleaning Validation in Ensuring Safe Product Changeovers
Managing cleaning validation manually can be complex. However, a robust digital cleaning validation solution like ValGenesis Process Manager, combined with ongoing cleaning processes management with ValGenesis e-Logbook, can dramatically enhance the safety and efficiency of your product changeovers.
Here’s how the ValGenesis Process Manager and ValGenesis e-Logbook can add value to your organization:
Automated Protocol Generation and Standardization
ValGenesis Process Manager eliminates the variability associated with manual cleaning validation. It allows manufacturers to develop standardized cleaning protocols that are generated automatically based on predefined parameters such as product type, equipment configuration, cleaning requirements, and risk levels. This standardization ensures consistency across all cleaning activities, reducing the risk of cross-contamination and procedural errors.
Ongoing Data Capture and Monitoring
A key benefit of a digital cleaning validation solution is continuous monitoring. Through cleaning log data, the system captures critical process parameters (CPPs), such as cleaning agent concentration, temperature, action, and time, allowing operators to track cleaning cycles continuously. This data-driven approach helps manufacturers optimize cleaning performance and identify potential issues before they escalate.
Comprehensive Documentation and Audit Readiness
Regulatory compliance relies heavily on thorough documentation of cleaning activities. ValGenesis Process Manager systematically records all relevant cleaning data and generates detailed validation reports that are easily accessible during audits. By organizing and storing this information digitally, manufacturers can ensure they are always audit-ready and compliant with FDA, EMA, ICH, and other regulatory guidelines.
Reduction of Human Error and Enhanced Accuracy
Manual data entry introduces the possibility of errors that can compromise the validity of cleaning validation efforts. ValGenesis Process Manager minimizes these risks by automating the calculation of critical cleaning parameters, such as residual limits. ValGenesis e-Logbook further reduces the risk of human error by digitizing log data entry, ensuring higher accuracy and reliability in validation.
Effective Risk Management
ValGenesis Process Manager’s built-in risk-based assessment tools help manufacturers prioritize high-risk areas of the cleaning process, enabling them to focus resources where they are needed most. The system can automatically assess whether critical cleaning validation steps are followed, ensuring no critical parameters are overlooked. This risk-based approach aligns with ICH Q9 guidelines and enhances overall operational efficiency.
Increased Operational Efficiency
Traditional cleaning validation processes are time-consuming and labor-intensive. ValGenesis Process Manager streamlines these processes, reducing the time required for validation activities while supporting the development and execution of optimized cleaning processes. ValGenesis e-Logbook facilitates faster turnarounds between product changeovers by effectively managing individual tasks and complex equipment, product, and cleaning process matrices. This increased efficiency leads to greater equipment availability, enhanced production output, and reduced downtime, ultimately driving profitability.
The Future of Cleaning Validation in Shared Equipment Environments
As pharmaceutical manufacturing becomes more complex, the importance of digital solutions for maintaining product safety and regulatory compliance grows. A robust digital cleaning validation product like ValGenesis Process Manager offers a comprehensive solution to the challenges of product changeovers on shared equipment. By automating cleaning protocols, ensuring accurate monitoring, reducing human error, and enhancing regulatory compliance, ValGenesis Process Manager delivers significant value to pharmaceutical manufacturers.
Given the high costs of noncompliance, recalls, and contamination, adopting digital solutions that streamline and enhance cleaning validation processes is essential for the pharmaceutical manufacturing industry. For a company aiming to protect its reputation, safeguard patient safety, and optimize operational performance, ValGenesis Process Manager is the key to staying ahead of the curve.
Want to learn more about cleaning validation? Watch the webinar featured below.
ValGenesis Process Manager: The Future of Cleaning Validation
Explore the future of cleaning validation with advancements in digitization and technology, and learn how to overcome traditional challenges while meeting increasingly stringent regulatory expectations with a science- and risk-based approach.
Cleaning Validation
Rui Almeida
Director - Consulting Services