During biopharma process development, there are often process changes that occur between non-clinical and clinical phases. When it comes to early development stages, Comparability Studies can be challenging.
If you are facing this type of problem, we’ve got the perfect reading for you! This blog post goes over the key aspects of developing solid comparability assessments in this development stage. Check this out!
The First Question is, When Should you do Comparability Studies?
Sometimes your process needs to face changes in the manufacturing process. And you need to assure one thing: is that your process continues to ensure the quality, safety, and efficacy of the final product. So, it becomes important to make Comparability Studies.
Also, it’s important to identify the impact of these changes on your final product. And for that, you should carefully evaluate all consequences for the product and define appropriate criteria.
There are many factors that can influence the comparability studies of your product, such as:
- The stage of product development.
- The availability of validated analytical procedures.
- The extent of product and process knowledge.
Today, let’s focus on the comparability studies on early development stages and dive into the main challenges.
So, What Happens When you do Comparability Studies on Early Development Stages?
Well, imagine that you have a camera, and you need to take a picture of your processes’ different phases.
The data you’ll have available at the early development stages is relatively small, so the photo you will take will be a little blurred. However, more information will become available at the late stages, so the images you’ll take become increasingly sharper.
And that’s where comparability studies become challenging: How could you make comparability studies if you need to compare two blurred pictures?
To help you with this, we have some key aspects that can help you in your comparability assessments.
The Importance of Having the Acceptance Criteria
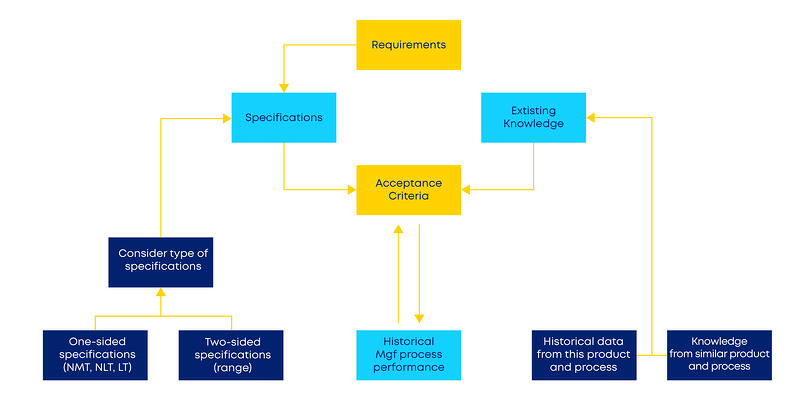
All comparability studies require acceptance criteria to guarantee that everything is accomplished during all the changes in the process. We can define these acceptance criteria according to:
- Specifications.
- Existing Knowledge.
When we talk about specifications, we should consider the different regulatory requirements that help you identify which type of tests you should do. There are two types of specifications:
- One-Sided Specifications.
- Two-Sided Specifications.
Also, we have the existing knowledge, which is essential for the definition of acceptance criteria. With that, you’ll be able to use the historical data from a product and processes or similars.
With the definition of these two parts, it’s fundamental to compare all process history performance and adjust them to every situation.
The key Aspects That Help Your Comparability Assessment
When it comes to the aspects that can help your comparability assessments, we should focus on the specifications. If you have a two-sided specification, the best approach to your comparability assessments will be Equivalence Testing.
On the other hand, if you have only one-sided specifications, you should opt for Non-Inferiority Testing and, eventually, a Superiority testing.