Control strategies are the backbone of product quality in the pharmaceutical industry, especially as regulatory expectations intensify. To remain compliant, efficient, and consistent, many manufacturers are turning to digital tools to enhance their control strategy process. By digitalizing, you not only streamline manufacturing but also achieve greater process control, reduce time to market, and ensure data transparency and integrity.
Here’s a roadmap to make the digital shift in your control strategy within the context of chemistry, manufacturing and controls (CMC).
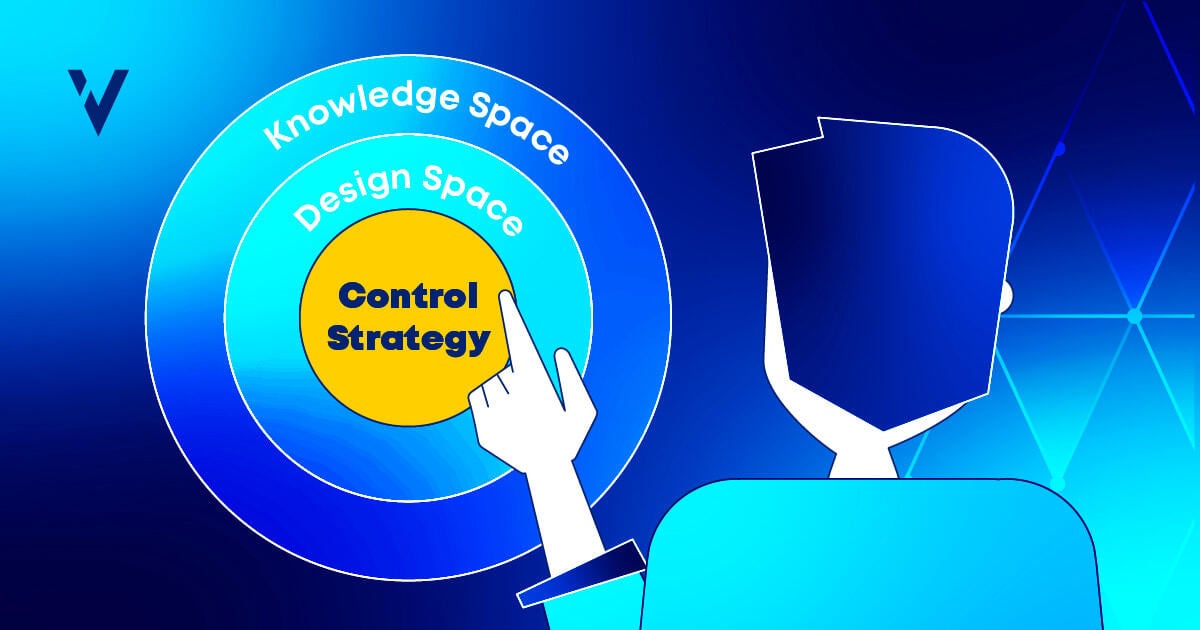
What is a Control Strategy?
A control strategy, as defined by the ICH Q10 guidelines, is a “planned set of controls derived from a deep understanding of the product and its process, ensuring both quality and consistency.”
You should build your control strategy by applying a Quality by Design (QbD) framework.
Quality by Design is a foundational approach that builds quality into products rather than relying solely on final product quality control (QC) testing. Regulatory guidelines, especially ICH Q8 through ICH Q12, strongly encourage QbD, emphasizing that product quality arises from scientific understanding and rigorous risk management.
Integrating QbD into your control strategy helps you identify and control potential quality issues early, making your processes more robust and adaptable to digital transformation.
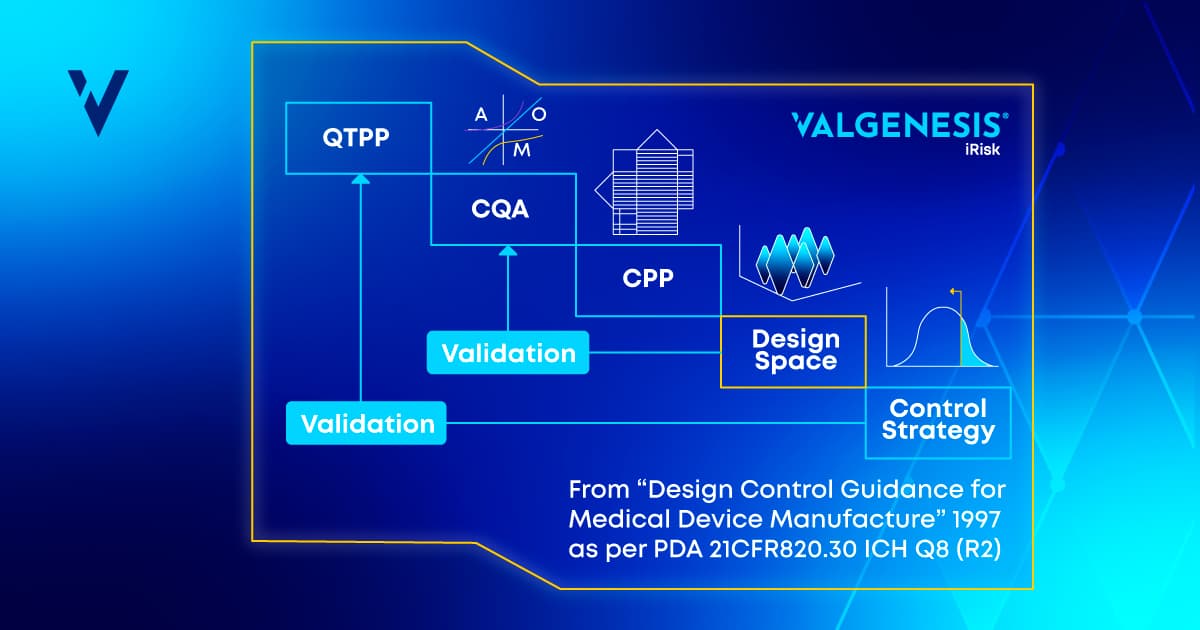
Quality by Design involves systematically designing processes to meet a quality target product profile (QTPP) by defining critical quality attributes (CQAs) and critical process parameters (CPPs) that ensure your product consistently achieves the expected quality outcome.
Here is a structured view of how QbD fits within a digital control strategy framework:
- Define the QTPP: Establish the desired quality characteristics of your product, including its clinical relevance, efficacy, and safety.
- Identify CQAs: Determine key product attributes that, if not met, could impact quality, efficacy, or safety.
- Determine CPPs: Specify the essential process variables that require thorough control to maintain CQAs within the necessary quality specifications and achieve the QTPP.
Defining the QTPP and identifying the CQAs and CPPs are part of an overarching process (see Figure 1 below) that ultimately should lead to the establishment of a process design space—a multivariate space where process parameters can vary within established limits while still yielding a product of desired quality. When defined digitally, the design space can be monitored in real time, allowing for adaptive controls and proactive responses to deviations to process behavior before they become a quality problem.

Figure 1: From QTTP to regulatory flexibility: a pathway to process excellence.
Digital QbD processes align well with modern control strategy goals by:
- Enhancing process understanding: Digital tools aggregate and analyze data, offering insights into how CPPs affect CQAs across different production stages and how to define scientific and risk-based product control strategies.
- Supporting real-time adjustments: By continuously monitoring the design space, digital systems can make prompt adjustments, helping to maintain the control state and, therefore, product quality.
- Facilitating compliance: Digital records provide easily accessible and comprehensive documentation, essential for regulatory reviews and audits.
By combining QbD and digitalization, control strategies become more efficient and adaptive, ensuring consistency from development throughout commercial manufacturing while adhering to industry standards and regulatory requirements.
Why Go Digital with Control Strategies?
Traditionally, control strategies involve intensive manual work, data entry, and numerous quality checks. This approach is slow, and when there is for example, a departure from the controlled state, the resulting adjustments are reactive. Digital control strategies offer a proactive solution and several advantages over traditional methods, including:
- Real-time monitoring: Digital tools allow real-time tracking of CQAs and CPPs, instantly detecting deviations to process behaviors.
- Enhanced data integrity: Digital data storage minimizes human error and reduces manual data manipulation.
- Process efficiency: Integrated digital systems streamline data collection, analysis, and storage.
- Reduced downtime: Automation reduces process delays and enables faster response times.
These improvements translate to lower costs, fewer errors, and, most importantly, more consistent product quality.
Regulatory Compliance in a Digital Framework
Regulatory bodies like the FDA and EMA require comprehensive documentation and quality assurance across the product lifecycle.
Regulatory compliance remains a primary concern in pharmaceutical manufacturing, with control strategies playing an essential role in meeting stringent guidelines. The ICH Q10 guideline describes control strategies as structured sets of controls designed to ensure product quality. When integrated into a digital framework, these strategies not only streamline compliance efforts but also enhance traceability, documentation, and process control—all pivotal for regulatory adherence.A digital control strategy framework offers several compliance-related benefits:
- Automated documentation and traceability: Digital systems record and store all relevant data from the manufacturing process, creating an audit trail that is easy to access and update. This ensures documentation aligns with regulatory standards without manual data entry errors or delays.
- Reporting and data integrity: Integrated data systems provide better reporting on CQAs and CPPs by enhancing overall data integrity—a key regulatory requirement.
- Continuous compliance monitoring: Through continued process verification (CPV) / ongoing process verification (OPV) and QbD, a digital control strategy maintains consistent quality standards and demonstrates an ongoing state of control, meeting FDA and EMA expectations.
- Faster regulatory approval processes: Automated and transparent data reporting simplifies the preparation and review of submissions.
- Enhanced audit readiness: Digital audit trails and real-time reporting ensure data is always up-to-date and easily accessible for inspections.
Steps to Digitalize Your Control Strategy
Phase 1: Assessment and Planning
Start by evaluating your current control systems. Assess risk and determine digital readiness across these areas:- Risk assessment: Identify and categorize risks associated with CQAs and CPPs.
- Gap analysis: Identify weaknesses in current processes that digital tools could address.
- Technology audit: Examine your current technology infrastructure to ensure it can support new digital initiatives.
- Goal setting: Define clear objectives for digitalization that align with regulatory and quality requirements.
Phase 2: Strategy Development
With clear goals, develop a strategy to integrate digital tools:- Technology selection: Choose digital solutions that meet your needs and can integrate with existing systems.
- Data strategy: Plan for secure and compliant data management, ensuring data integrity and access control.
- Process design: Redesign processes to support automation and real-time monitoring.
Phase 3: Implementation
Begin by rolling out digital initiatives through pilot projects to test scalability:- Pilot projects: Implement small, manageable pilot programs to test digital tools.
- Scale-up: Evaluate pilot results and then scale successful digital initiatives across broader production.
- Change management: Apply change management strategies to ensure all staff are well-trained on new digital processes.
Phase 4: Continuous Improvement
Once implemented, focus on ongoing performance monitoring and adjustments:
- Performance tracking: Regularly measure key performance indicators (KPIs) to assess the effectiveness of your digital solutions.
- Feedback loops: Establish mechanisms to capture user feedback for continuous improvement.
- Adapt and refine: Adjust your digital control strategies based on new data insights or changes in regulatory standards.
CPV and Its Role in Control Strategies
Continued process verification (CPV) is the third stage in process validation, focusing on ensuring that a process remains in control during its commercial phase.
Regulatory bodies, such as the FDA, emphasize CPV as a mechanism to confirm that manufacturing processes consistently yield quality products. This makes CPV a critical aspect of any control strategy, emphasizing the monitoring of process performance over time to ensure product quality remains within specifications.
Continued process verification enables ongoing verification of the CPPs and CQAs established during initial process design, which are part of the control strategy.
The data collected through CPV provides a feedback loop that informs adjustments to the control strategy, refining processes based on actual production data. This closed-loop approach aligns well with QbD, enhancing process understanding over time.
The Digital Approach to CPV Itself
With a digital approach, CPV becomes more manageable, yielding insights for proactive adjustments rather than retrospective corrections.
A digital approach to CPV strengthens control strategies in several ways:
- Improved process robustness: Real-time data and trend analysis help identify issues before they impact product quality, allowing for quicker, targeted responses.
- Enhanced compliance: Digital CPV provides a comprehensive audit trail, making it easier to demonstrate ongoing process control during regulatory audits.
- Reduced product testing: With consistent data and process stability, and based on a risk-based approach, manufacturers can minimize end-product testing, potentially enabling real-time release.
Integrating a digital CPV tool into a digital control strategy ensures that your process maintains a state of control, adapting to process variability as tit arises.
The two main advantages of integrating a digital CPV tool into the digital management of the control strategy are:
- Data collection and integration: Digital tools gather data across production lines, capturing process parameters and quality metrics in real time. This integrated data flow is essential to track consistency and spot early trends that indicate potential deviations in process behavior and performance.
- Real-time monitoring: Automated monitoring systems evaluate CPPs and CQAs continuously, which enables the manufacturing process to operate within the design space. If variations occur beyond predefined conditions, CPV tools can trigger alerts for immediate corrective actions.
This approach helps sustain product quality throughout the product lifecycle, aligning with regulatory requirements and supporting efficient, data-driven process management.
Key Technologies for Maintaining a State of Control
Several digital technologies, combined with digital control strategies and digital CPV software, support maintaining a state of control in processes:
- Process analytical technology (PAT): Enables real-time monitoring of CQAs to ensure products meet quality benchmarks.
- Internet of things (IoT): Connects manufacturing equipment for continuous data collection and communication on key parameters such as temperature, pressure, and humidity.
- Machine learning (ML) and artificial intelligence (AI): Based on advanced statistical algorithms, these tools analyze large datasets to identify trends and predict potential issues before they occur.
- Digital twin technology: Creates a real-time digital replica of your process, allowing you to simulate and optimize processes virtually before implementing changes physically.
Overcoming Challenges with Digital Control Strategies
Digitalizing control strategies offers clear benefits, but it also presents challenges. Implementing a digital approach requires a shift in infrastructure, processes, and potentially company culture.
Here are some common challenges and solutions:- Data silos: Integrate data systems to avoid isolated data points.
- Obtaining employee buy-in: Train employees on digital tools and demonstrate their time-saving benefits.
- Cost of implementation: Start with pilot projects to showcase ROI before scaling up.
Moving Forward with Digital Control Strategies
Adopting a digital control strategy is more than an operational upgrade; it’s a commitment to quality, compliance, and innovation in pharmaceutical manufacturing. Digitalizing control strategies transforms complex processes, enabling real-time monitoring, automated documentation, and seamless regulatory compliance.
By combining QbD, CPV, and data-integrated systems, digital control strategies shift the quality paradigm from reactive to proactive. This approach allows you to address risks early, maintain product consistency, and support continuous improvement.
Beyond meeting regulatory standards, digital control strategies provide a framework for efficiency, cost savings, and faster time-to-market. Real-time data insights, automated reporting, and an adaptive process design space empower you to manage every aspect of production with precision. By moving away from manual, paper-based systems, your team can focus on process optimization, reduce reliance on end-product testing, and pave the way for real-time release.
Curious to learn more? Watch the webinar to explore this topic in depth and gain valuable insights that can elevate your understanding!
A Roadmap to Digitalize Your Control Strategy
Learn essential steps for digitalizing control strategies in the pharmaceutical and biopharmaceutical industries, and explore key concepts and benefits of digital solutions.
Digital Transformation Process Digitalization
Daniel Pais
Senior Consultant