The pharmaceutical industry is undergoing a transformative shift driven by the integration of data and digital technologies. As companies strive to streamline processes and enhance product quality, adopting specialized tools offers many advantages.
In this blog post, we will explore how one tool, ValGenesis iRisk, stands out as the most comprehensive platform for applying the principles of Quality by Design (QbD) and Analytical Quality by Design (AQbD). We will also discuss the advantages of digitalization in pharmaceutical development and how ValGenesis iRisk is revolutionizing the industry.
Challenges in Pharmaceutical Development
Pharmaceutical development traditionally involves manual processes, paper-based documentation, and fragmented data systems. This outdated approach leads to inefficiencies, disengagement among skilled professionals, and delays in product development and regulatory approval. As the International Council for Harmonization (ICH) guidelines emphasize, particularly ICH Q8, Q9, Q10, Q11, and Q14, there is a pressing need for a harmonized, systematic approach to ensure product quality and compliance throughout the lifecycle.
The industry's reliance on manual risk assessments and disparate knowledge sources further complicates the implementation of QbD frameworks. This fragmentation often results in inconsistent application of risk management strategies, increased time and costs for Chemistry, Manufacturing, and Controls (CMC) deliverables, and challenges in leveraging existing knowledge.
What about ValGenesis iRisk?
ValGenesis iRisk addresses these challenges by providing a robust digital platform that integrates QbD and AQbD processes. It offers a structured approach to identifying critical quality attributes (CQAs), critical process parameters (CPPs), and critical material attributes (CMAs). This platform supports the development of control strategies, ensuring that all aspects of the pharmaceutical development process align with regulatory requirements.Key features of ValGenesis iRisk include:
- Pre-defined Workflows and Templates: iRisk provides standardized workflows and templates, enabling consistent application of QbD principles across all stages of product development. This standardization reduces the variability in risk assessments and ensures a uniform approach to product quality.
- Cross-Functional and Cross-Company Cooperation: The platform facilitates collaboration among stakeholders. By providing a centralized knowledge base, iRisk enhances communication and ensures that all parties have access to the latest data and assessments.
- Automated Knowledge Management: ValGenesis iRisk centralizes data storage and retrieval, making it easier to access and manage information. This automation reduces the need for manual transcription and minimizes the risk of errors, streamlining the entire data management process.
- Flexible Reporting and Visualization: The platform generates automated charts, process maps, and reports tailored to specific needs. This flexibility in reporting enhances transparency and aids in decision-making, facilitating faster regulatory submissions and approvals.
- Integration with Existing Systems: iRisk integrates seamlessly with systems such as Laboratory Information Management Systems (LIMS), Electronic Document Management Systems (EDMS), and Quality Management Systems (QMS). This integration streamlines information flow and reduces redundant efforts.
- Comprehensive Risk Tools: The platform includes a variety of risk assessment tools, allowing for thorough analysis and comparison of different risk scenarios. This comprehensive approach enables more effective identification and prioritization of risks, leading to better-informed control strategies.
Case Studies and Real-World Applications
Case Study #1: Streamlining the QbD Framework in CMC Development
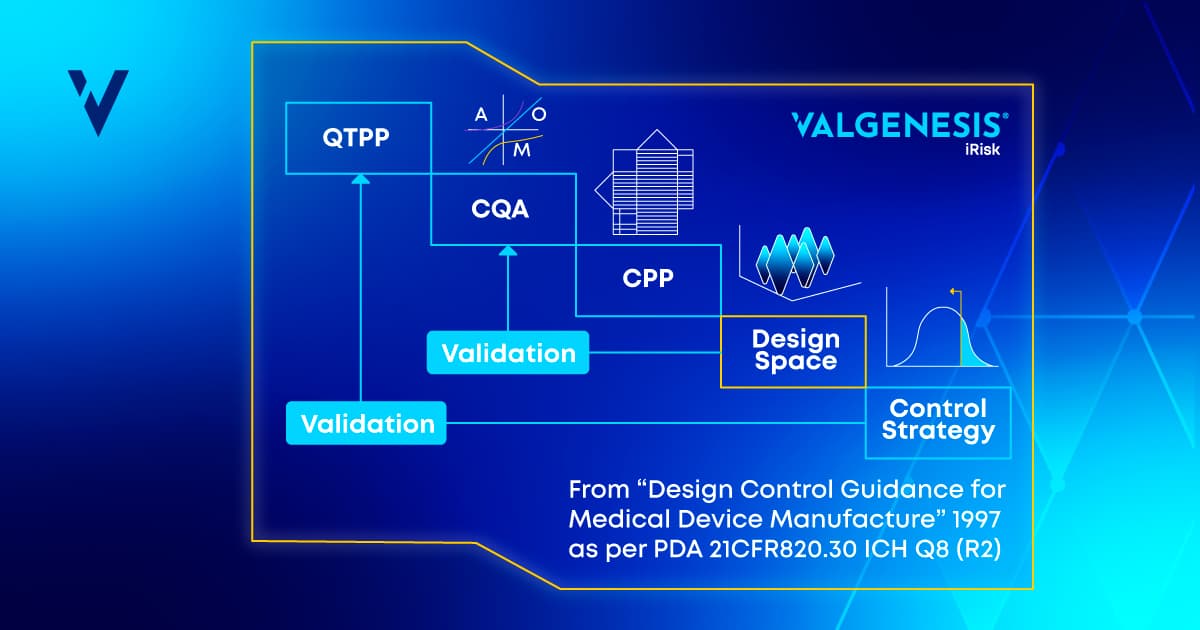
A leading pharmaceutical company struggled with managing complex QbD data flows scattered across multiple systems and documents. By implementing ValGenesis iRisk, the company significantly improved its management of CQAs and CPPs through the following workflow:- Definition of Quality Target Product Profile (QTPP) and Critical Quality Attributes (CQAs): The process began by listing the product’s quality attributes in relation to the QTPP. Attributes without defined criticality were evaluated based on their impact on product quality and the level of uncertainty in their criticality. Each attribute was assigned a value, and a final score was calculated to determine the criticality of each quality attribute.
- Assignment of Process Parameters Criticality: The next step involved establishing the connection between identified quality attributes and the corresponding process parameters. This step created a correlation matrix to assess the impact of each process parameter on specific attributes, which facilitated the determination of the parameters' criticality.
- Further Assessment: At a later stage of product development, a product risk assessment was conducted using previous tools. This identified specific areas requiring attention in the Failure Mode and Effects Analysis (FMEA) process. Mitigation actions were then incorporated to reduce risks to acceptable levels.
- Checkpoint for Criticality Revision and Automated Control Strategy: Digital tools were employed to identify inconsistencies and compile a list of inputs and their respective criticalities. This step served as a focal point for revising assigned criticalities based on the conducted risk assessments. Finally, a control strategy was automatically generated based on the inputted risk information, ensuring a comprehensive approach to managing product and process risks.

Figure 1: QbD risk management workflow
ValGenesis iRisk facilitated regulatory compliance by providing a comprehensive understanding of product and process risks. This thorough documentation enhanced regulatory submissions, reduced the likelihood of regulatory hurdles, and expedited approval timelines. The platform also accelerated development timelines by streamlining risk assessment processes and optimizing experimental designs, aiding in the rapid development of robust control strategies.
Additionally, ValGenesis iRisk enhanced knowledge sharing within the organization by centralizing information related to quality risk management exercises for specific products. This centralized approach minimized the loss of critical knowledge and prevented redundant efforts. Moreover, the platform expedited reporting by enabling the effortless generation of essential documents, such as CQA assessment reports and Control Strategy Summary reports. This efficiency prevented delays in submitting CMC regulatory documentation, supporting smoother regulatory processes.
Case Study #2: Enhancing Analytical Method Development Using an AQbD Framework
ValGenesis iRisk was also utilized for AQbD applications. The platform enabled the definition of an Analytical Target Profile (ATP) and the identification of critical method attributes (CMAs) and critical method parameters (CMPs).The following steps were taken:
- Definition of Critical Quality Attributes: A list of quality attributes was gathered and evaluated for criticality using specific criteria. This involved a scoring process that considered both impact and uncertainty, setting the foundation for the subsequent steps in the strategic workflow.
- Linking Product Development to Process Understanding: The strategy moved to understanding the criticality of process parameters and the potential for introducing failure modes. Required mitigations were integrated to address these risks.
- Information Consolidation: All previously gathered information was consolidated to define the Analytical Target Profile (ATP).
- Analytical Workflow Establishment: The complete list of attributes from the consolidation phase guided the selection of the best analytical technology for each specific quality attribute. This phase culminated in the development of methods, with specific method parameters being established and their criticality determined.

Figure 2: Connection between process and analytical workflow
ValGenesis iRisk automated routine tasks, reducing manual efforts and accelerating decision-making processes. The digital tool provided a dynamic platform that enhanced communication and collaboration among the diverse teams involved in quality risk management. Through cloud-based solutions, stakeholders had access to risk assessments and could actively contribute to them, promoting transparency and a collective approach to addressing potential issues.
Additionally, the platform enhanced reporting capabilities by offering flexibility in creating reports tailored to specific needs and preferences. It enabled timely reporting through the use of configured templates adapted to the company’s requirements, streamlining the documentation process and ensuring that relevant information was readily available.
The Impact of Digitalization with ValGenesis iRisk
The adoption of ValGenesis iRisk leads to numerous benefits, including:- Enhanced Product Quality: By systematically applying QbD principles, companies can design robust manufacturing processes and develop effective control strategies. This ensures consistent product quality and compliance with regulatory standards.
- Resource Optimization: iRisk allows for better resource allocation by identifying high-risk areas that require focused attention. This targeted approach optimizes the time and costs associated with CMC deliverables.
- Regulatory Compliance: The platform's comprehensive documentation and reporting capabilities facilitate smoother regulatory interactions, reducing the likelihood of compliance issues and speeding up approval processes.
- Knowledge Preservation and Sharing: The centralized knowledge management system prevents the loss of critical information and promotes knowledge sharing across departments and organizations. This ensures continuity and consistency in product development efforts.
Conclusion
Digital transformation is reshaping industries, and the pharmaceutical sector must embrace these changes to remain competitive and compliant. ValGenesis iRisk offers a powerful solution that integrates QbD and AQbD processes into a single, cohesive platform fully applicable to pharmaceutical development.
By leveraging the advanced features of iRisk, pharmaceutical companies can enhance their product development processes, improve quality, and achieve faster regulatory approvals. Embracing digital platforms like ValGenesis iRisk is not just a step toward modernization but a critical move to ensure the continuous delivery of safe and effective pharmaceutical products in a rapidly evolving market.
For more details, visit the ValGenesis iRisk page.