This post is the second installment of a two-part series on best practices for an integrated commissioning and qualification (C&Q) approach. The first installment examined phases one (Requirement) and two (Design), C&Q planning, and the rationale behind integrating commissioning and qualification activities. In this final installment, we look at phases three (Verification) and four (Quality Decision) and reveal the secret ingredient to C&Q success.
Read part one before delving into part two to fully grasp the concepts discussed in this post.
C&Q Phases and Best Practices
To recap, C&Q is an intricate process. Its complexity often leads to confusion about the necessary steps to take and when to take them. An integrated approach based on science and risk can simplify C&Q activities, making the process more efficient, compliant, and cost-effective.
.jpg?width=1200&height=603&name=1200x603-diagram%20(2).jpg)
Figure 1: The suggested framework for the risk-based implementation of integrated C&Q.
An integrated C&Q approach comprises four phases: requirement, design, verification, and quality decision. We've already discussed phases one and two. Let's move on to phase three.
Phase Three: Verification Phase
Phase three concerns testing and documentation. C&Q testing and documentation spans from design to system release. The scope of the testing depends on the scope of the project. The type and rigor of testing depend on the type of system (or equipment) and its potential to impact product quality. Key verification activities include:
- Planning: This involves establishing a comprehensive plan for verification activities, outlining the scope, schedule, resources, and responsibilities for testing and documentation. The verification plan serves as a roadmap, guiding the execution of verification activities and ensuring that all necessary tests are conducted to demonstrate compliance with requirements.
- Training: It's important that personnel are adequately trained in verification procedures and documentation standards to execute verification activities effectively. Training programs should cover testing protocols, data recording practices, safety procedures, and regulatory compliance requirements.
- DQ integration: Integrating design qualification into the testing process ensures the system design meets predefined requirements and specifications before verification activities begin. DQ activities may include reviewing design documents, conducting risk assessments, and verifying design specifications. By incorporating DQ into the verification phase, potential design flaws or deficiencies can be identified and addressed early in the process.
- Vendor documentation and testing: Using vendor documentation and testing results leverages the expertise and resources of equipment manufacturers or suppliers. Vendor documentation may include specifications, test reports, and validation protocols you can use to support qualification activities. Incorporating vendor testing results minimizes redundant testing efforts, speeding up qualification.
- Change management: Managing system changes through established engineering change management procedures is essential for maintaining the integrity and compliance of the verification process. Any changes to system design, specifications, or requirements should be carefully evaluated, documented, and approved through a formal change control process. Effective change management helps prevent unauthorized modifications, ensures traceability of changes, and minimizes the impact on verification activities.
- Execution: Carrying out various testing and execution activities involves executing the verification plan, including vendor and system construction phase documentation, installation testing, startup execution, operational and functional testing, and performance qualification. Execution activities require meticulous attention to detail, adherence to established procedures, and accurate documentation of test results and observations.
- Deviation or exception management: Implementing procedures to manage and address deviations or exceptions encountered during verification ensures that non-conformances are identified, documented, and resolved promptly. Deviations may arise due to unexpected test results, equipment malfunctions, or procedural errors. Effective deviation management involves investigating the root cause of deviations, implementing corrective actions, and documenting the resolution process to maintain compliance with requirements.
Phase Three Best Practices
Before commencing verification activities, it's crucial to establish clear and consistent standards for documentation. This includes defining formats, templates, naming conventions, and version control protocols for all verification-related documents. Adhering to predefined documentation standards ensures consistency, clarity, and traceability throughout the verification process.
Other best practices include:
- Train on documentation standards: Once you establish documentation standards, providing comprehensive training to relevant personnel is essential. Training should cover the specifics of the documentation standards, including how to create, review, revise, and approve verification documents. Training sessions may include workshops, seminars, or online modules to ensure all team members understand and adhere to the established standards.
- Predefine test witnesses: Test witnesses play a crucial role in verification activities by providing oversight, verification, and validation of test procedures and outcomes. Identifying key test witnesses and associated requirements beforehand ensures that the right individuals are involved in each verification test. These individuals should possess the necessary expertise, authority, and independence to evaluate test results and provide validation impartially.
- Assess the vendor: When leveraging vendor documentation and testing results for the validation turnover package (VTOP), it's essential to conduct a thorough audit or review of the vendor's processes. This assessment ensures that the vendor's documentation meets the required quality standards and regulatory requirements.
- Manage system changes: Throughout the verification phase, changes to the system design or specifications may occur. It's crucial to establish a robust system for managing these changes effectively. This includes implementing formal engineering change management processes to document, evaluate, approve, and implement any proposed changes. By following predefined change management procedures, you can minimize potential disruptions or delays to the verification process and preserve the integrity of the qualification results.
- Predefine the process for test failures: Despite thorough planning and execution, test failures may occur during the verification phase. To address these failures promptly and effectively, it's essential to establish a predefined process for managing test failures. This process should include procedures for promptly identifying, documenting, investigating, and resolving test failures. By having clear escalation pathways, corrective actions, and decision-making protocols in place, teams can expedite the resolution of test failures and maintain project momentum.
Phase 4: Quality Decision Phase
The last phase in our C&Q journey is the Quality Decision Phase. The key elements of this phase include:
- Prerequisites completion: Ensure all prerequisites, including documentation and testing, are met before proceeding.
- Investigation of unexpected results: Investigate any unexpected or anomalous results encountered during testing.
- Addressing discrepancies/deviations/exceptions: Address and resolve any discrepancies, deviations, or exceptions identified during the verification phase.
- Closure of engineering change management (ECM): Close out all engineering change management activities related to the project.
Phase Four Best Practices
Ensuring that test descriptions are clear and comprehensive is vital. Each test should be accompanied by a detailed description outlining the expected results, criteria for success, references, and actual results obtained. Additionally, the outcome of the test, whether it passed or failed, should be explicitly stated. Providing such clarity facilitates the review process for quality personnel, enabling them to make informed decisions regarding acceptance and release.
Other best practices include:
- Manage discrepancies/exceptions: When you identify discrepancies or exceptions, investigate and address them promptly. Discrepancies should be managed to closure following established procedures, such as engineering change control or formal quality change control processes. Proper handling of discrepancies ensures that quality standards are upheld and mitigates potential risks to the system's integrity and functionality.
- Implement execution metrics: Implementing robust execution metrics that track passed, failed, and deviated outcomes is crucial for expedited decision-making. These metrics provide a comprehensive overview of the C&Q project's execution status, highlighting areas of compliance and noncompliance. By having access to clear and concise execution metrics, quality personnel can assess the project's progress and identify any immediate issues, facilitating efficient decision-making processes.
- Ensure availability of quality system elements: All necessary quality system elements should be in place before system release. This includes documentation, procedures, and controls that ensure product quality and regulatory compliance. By ensuring the availability of quality system elements, organizations can demonstrate readiness for system release and mitigate the risk of quality-related issues post-deployment.
- Prepare formal summary with approval: A formal summary of key project deliverables, such as qualification reports, should be prepared and approved before system release. This summary is a comprehensive overview of the project's outcomes, highlighting key findings, actions taken to address discrepancies, and overall compliance status. Obtaining formal approval ensures that stakeholders agree on the project's readiness for release.
Best Practices for C&Q Projects
In the broader context of the entire C&Q project, several overarching best practices contribute to its success. These include:
- Inclusive design review: Include all C&Q engineers, site personnel, vendors, and consultants in the design review process to ensure comprehensive input and alignment with project goals.
- System categorization: Drive the C&Q project using system categorization to ensure standardized and efficient execution tailored to specific system requirements. Your C&Q efforts should not be person-driven or function-driven work; a standard system should drive them.
- Timely change management: Delay formal change control requests (CCRs) until after qualification or design qualification phases to avoid unnecessary delays and streamline project progression. ISPE also supports a realistic approach to change management.
- Focused testing: Prioritize critical function and aspect testing during IQ or OQ (where applicable) to ensure a thorough evaluation of system performance and compliance.
- Leveraging testing documents: Execute testing documents wherever possible by subject matter experts (SMEs) and leverage them for qualification to optimize resources and ensure accuracy. Vendors can also be considered SMEs.
- Clear RACI Matrix: Establish a well-accepted RACI (Responsible, Accountable, Consulted, Informed) matrix to clarify roles and responsibilities throughout the project, ensuring efficient collaboration and decision-making.
- Trust-based collaboration: Foster a culture of trust among project stakeholders to facilitate seamless communication, decision-making, and project execution. A trust-based process is the backbone of successful C&Q.
- Digitalization: Embrace digital solutions like ValGenesis to enhance project efficiency, automate processes, and ensure compliance. A robust digital system should drive system categorization, manage engineering changes and deviations, automate trace matrix generation (Figure 2), and facilitate time-bound requalification efforts post-handover, minimizing human intervention and optimizing resource utilization.
Digitalization is the secret ingredient to achieving your C&Q goals faster and more compliantly with limited resources!
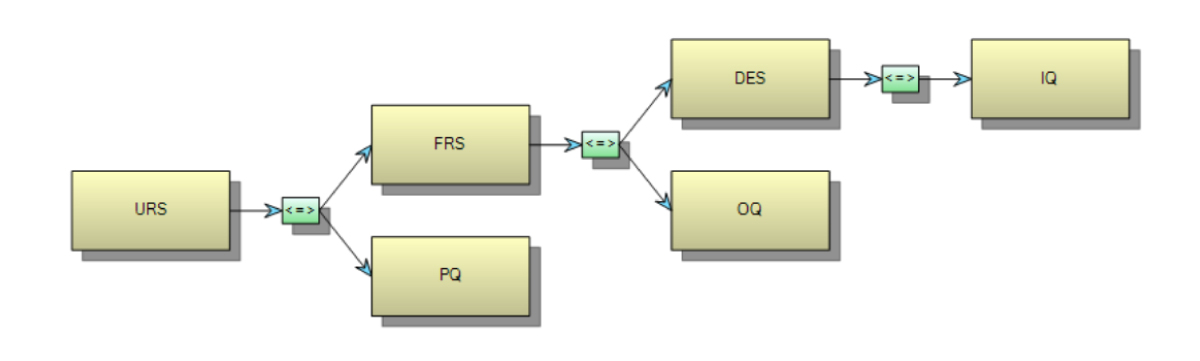
Figure 2: Trace matrix generated automatically in ValGenesis minimizes the human error associated with manual trace matrix creation and Excel.
Key Takeaways
Integrating commissioning and qualification processes is intricate yet crucial for ensuring the integrity, safety, and compliance of critical systems in regulated industries like pharma and biotech. From the initial requirement phase through design and verification and into the quality decision phase, each step demands meticulous planning and execution.
By following the best practices presented in this blog series and leveraging innovative digital technologies like ValGenesis, regulated organizations can streamline their C&Q processes, mitigate risks, and achieve their project goals effectively.
Note: This blog series is based on the “Best Practices in Commissioning and Qualification" webinar hosted by Saurabh Joshi, Director of Industry Solutions at ValGenesis. For a deeper dive into this topic, watch the webinar.