Avoiding Common FDA 483 Observations in Cleaning Validation


In the highly regulated world of pharmaceutical manufacturing, compliance with FDA guidelines is not just a legal requirement, it's a critical component of ensuring patient safety and product efficacy. One area that receives significant scrutiny during FDA inspections is cleaning validation, a process that ensures all traces of contaminants are removed from manufacturing equipment. Failing to meet the FDA's stringent standards can lead to 483 observations, which are often precursors to more severe regulatory actions.
Building on a previous blog post about the regulatory expectations for cleaning validation, this post explores some common causes of these observations and provides actionable strategies to help manufacturers stay compliant.
Understanding FDA 483 Observations
FDA 483 observations are issued when an FDA inspector identifies conditions that may violate the Food, Drug, and Cosmetic (FD&C) Act. Although these observations are not final determinations of noncompliance, they are serious enough to warrant attention and corrective action. If left unaddressed, they can escalate to Warning Letters, consent decrees, or even criminal charges, significantly impacting a company's operations and reputation.
The Critical Role of Cleaning Validation
Cleaning validation is the cornerstone of maintaining product safety in pharmaceutical manufacturing. It involves proving that the cleaning procedures in place effectively remove contaminants to a predefined, acceptable level. This process is essential not only for regulatory compliance but also for ensuring that products are free from cross-contamination and safe for consumer use.
However, the complexity of modern pharmaceutical manufacturing, combined with stringent regulatory requirements, makes cleaning validation a challenging task. This is where many companies falter, leading to FDA 483 observations.
Common Causes of FDA 483 Observations in Cleaning Validation
- Inadequate Cleaning Validation Protocols: Many companies fail to develop comprehensive cleaning validation protocols. These protocols should detail every step of the cleaning process, including the methods used, the equipment involved, and the specific parameters to be monitored. A common mistake is the lack of clearly defined acceptance criteria, which makes it difficult to determine whether a surface is truly clean. Without these criteria, operators may inconsistently apply cleaning procedures, leading to variability and potential contamination.
- Insufficient Cleaning Validation Studies: Insufficient validation studies are another major cause of FDA 483 observations. These studies must cover a broad range of scenarios, including worst-case conditions where equipment is exposed to the highest levels of contaminants. However, many companies conduct only minimal studies, failing to account for process variability or the full range of products and equipment used in manufacturing. This oversight can result in a false sense of security regarding the effectiveness of cleaning procedures.
- Failure to Establish Cleaning Limits: Establishing and justifying residue limits is crucial for effective cleaning validation. These limits must be based on sound scientific principles, ensuring that any remaining residues do not pose a risk to product safety or efficacy. Unfortunately, some companies set limits without proper justification or fail to include an adequate margin of safety. This oversight can lead to unsafe levels of contamination, ultimately compromising product quality.
- Inadequate Monitoring of Cleaning Procedures: Monitoring is essential to ensure that cleaning processes remain effective over time. However, inadequate or inconsistent monitoring is a common issue identified by the FDA. This includes failing to conduct periodic revalidation, which is necessary to confirm that cleaning procedures continue to work as intended as conditions change. Additionally, poor documentation of monitoring activities can make it difficult to demonstrate compliance during inspections.
- Inadequate Documentation and Change Control: Documentation is a fundamental aspect of cleaning validation, yet it is often neglected. Inadequate record-keeping, such as missing or illegible records, can lead to significant compliance issues. Similarly, poor change control practices can result in gaps in validation, especially when changes are made to the manufacturing process or equipment without proper documentation.
- Environmental Monitoring: Environmental monitoring is a complex but critical aspect of maintaining compliance, particularly in manufacturing facilities. The FDA places significant emphasis on environmental monitoring, especially in sterile environments. It is essential to establish and implement a comprehensive environmental monitoring program across all classified areas of the facility. This program should include regular monitoring at scheduled intervals to ensure ongoing compliance and environmental control.
- Lack of Risk Assessment: Inadequate risk assessment in cleaning validation can lead to missed opportunities to systematically identify and mitigate potential issues that could compromise cleaning effectiveness. This oversight can result in insufficient validation of cleaning processes, particularly in worst-case scenarios or with challenging residues. Conducting thorough risk assessments is crucial for establishing appropriate acceptance criteria and prioritizing validation activities. Without it, the likelihood of product contamination and regulatory noncompliance increases. Continuous risk evaluation is essential to ensure that cleaning processes remain effective as conditions evolve.
Strategies for Avoiding FDA 483 Observations
- Develop Comprehensive Cleaning Validation Protocols: Start by developing detailed cleaning validation protocols that cover all aspects of the cleaning process. Clearly define acceptance criteria and base them on scientific evidence. Regularly review and update these protocols to reflect changes in manufacturing processes or regulatory expectations.
- Conduct Thorough and Comprehensive Validation Studies: Validation studies should be thorough and cover a wide range of scenarios, including worst-case conditions. Ensure these studies account for all products, equipment, and process variations that could impact cleaning effectiveness. This approach helps identify potential issues before they lead to regulatory observations.
- Establish and Justify Residue Limits: Residue limits must be scientifically justified and include an adequate margin of safety. Use a risk-based approach to set these limits, considering factors such as the toxicity of the residue, the batch size, and the dosage of the final product. Regularly review and update these limits as new information becomes available.
- Enhance Monitoring and Documentation Practices: Implement robust monitoring procedures to ensure that cleaning processes remain effective over time. This includes conducting periodic revalidation and thoroughly documenting all monitoring activities. Use digital tools, such as the ValGenesis Validation Lifecycle Management System (VLMS), to streamline documentation and ensure data integrity.
- Improve Change Control Processes: Strengthen change control processes to ensure that the impact of any change to product, process, or equipment is adequately assessed, documented, and validated. This will help prevent gaps in cleaning validation and reduce the risk of FDA 483 observations.
- Enforce Area Cleaning Practices: Automate the scheduling of regular cleaning and sanitation activities to ensure adherence to standardized procedures. Systematically enforce the use of appropriate forms or checklists, supported by documented evidence of completed tasks. Digital solutions like ValGenesis iOps™ can efficiently manage all cleaning and sanitation logs, ensuring thorough area monitoring and compliance.
- Execute and Track Mitigation Plans: Leveraging a powerful digital tool like ValGenesis iClean™, customers can systematically assess risk and enforce all required mitigation steps, tracking action items during cleaning validation studies while ensuring compliance contemporaneously. (Watch the video below to see how digitalizing cleaning validation can support ALCOA+ and GMP cleaning validation standards.)
It Matters Beyond Simple Regulatory Compliance
Cleaning validation goes beyond merely satisfying regulatory requirements—it's a vital component of safeguarding patient health, ensuring product efficacy, and protecting business continuity.
Effective cleaning processes prevent cross-contamination, which not only protects consumers from potential harm but also preserves the integrity of the manufacturing process. By maintaining rigorous cleaning validation standards, manufacturers can avoid costly product recalls that damage both reputation and profitability. This commitment to quality strengthens trust with regulators, enhances company reputation, and ensures long-term success in a competitive market.
Ultimately, robust cleaning validation ensures that every product reaching the patient is safe, pure, and effective. Leveraging digital solutions, such as ValGenesis iClean™ can further enhance a company’s ability to address these challenges in a fully digital, scalable platform.
Related Blog Posts

Best Practices for Impact Assessments in Cleaning Validation
Explore best practices for impact assessments in cleaning validation, including strategies for using technology to streamline the assessment process.
By Sachin Maled
Read
How Can Digital Cleaning Validation Improve Product Changeover Safety?
Learn how digital cleaning validation solutions can prevent cross-contamination and ensure drug safety during product changeovers in pharma manufacturing.
By Kenneth Pierce
Read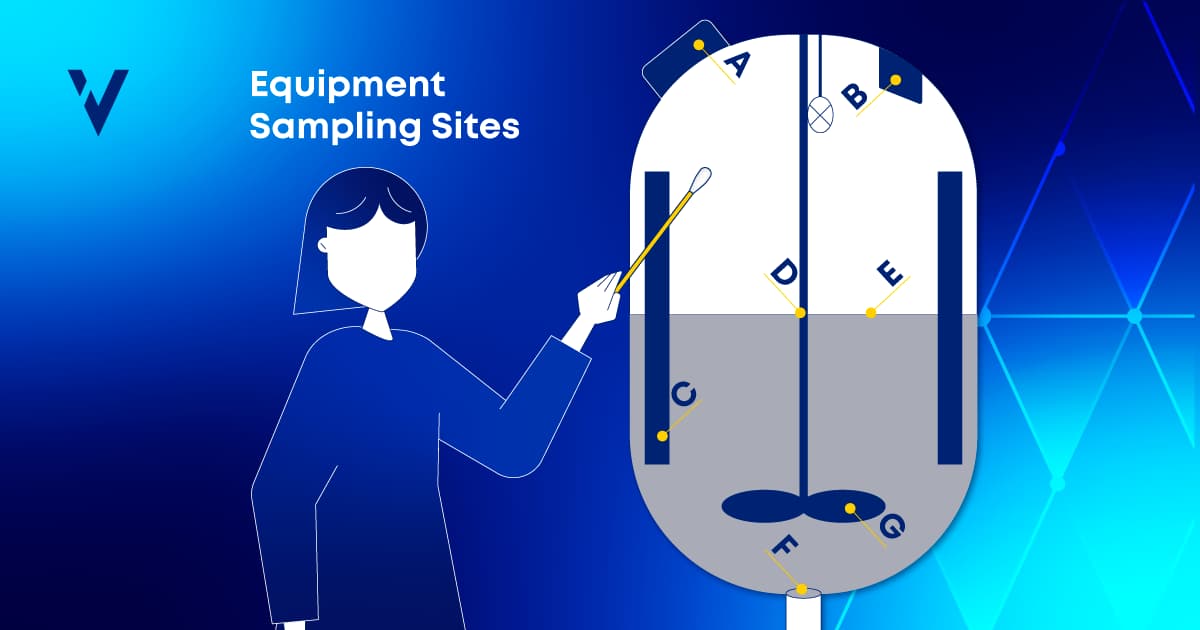
Equipment Design in Cleaning Validation: Enhancing Your Sampling Plan
Discover the importance of equipment design in cleaning validation and how ValGenesis Process Manager improves sampling plans and ensures compliance.
By Peter Liang
Read